Flame Retardant Mechanism
The flame retardant mechanism of aluminum hydroxide is relatively complex, consisting of the following synergistic mechanisms:
(1) Endothermic Reaction
Aluminum hydroxide reacts upon heating to produce aluminum oxide and water. This reaction is endothermic, with the reaction equation being:
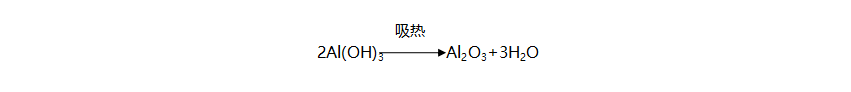
During this process, not only does it absorb heat and delay the combustion of the polymer, but the water vapor released also dilutes the flammable gases and oxygen, and participates in reactions in the condensed phase.
(2) Dilution Effect
In addition to the water vapor mentioned in (1) that dilutes flammable gases and oxygen, aluminum hydroxide also acts as a filler, reducing the amount of polymer per unit volume, thus providing a dilution effect.
(3) Covering Effect
The aluminum oxide produced by the reaction, along with other carbides, forms a flame-retardant barrier, inhibiting the spread of flames.
(4) Carbonization Effect
Under combustion conditions, the flame retardant produces strongly dehydrating substances, causing the plastic to carbonize and making it less likely to produce flammable volatile substances, thus preventing the spread of flames.
Advantages
(1) Prominent Endothermic Cooling Effect
(2) Environmentally friendly and safe during the flame retardant process, without producing toxic gases
(3) Reinforcing effect on rubber and other products
(4) Abundant Resources